In this day of pessimism and cynicism, it’s remarkable how uniformly positive news on cell therapies is. I’ll summarize: it’s going to save us all and the world. This article is not about debunking all that optimism. It merely aims to offer some pointers so we can be saved a bit faster.

So what are cell based therapies? Let’s exemplify using immunotherapy.
Our immune system has 2 jobs: detecting threats and eliminating them. In that order, as it needs to know something is up before it can take appropriate action. However, some diseases, such as cancer, are good at hiding from our immune system. This is why we need to take action ourselves: actively searching for malignant cells, and then removing those cells if they are accessible or trying to kill them with radiation or chemotherapy.
Unfortunately, our current treatments have severe side effects and are not 100% successful. So, as it is in our nature to improve, people start researching other, better ways to battle cancer. Some have asked themselves: what if we could make our immune system smarter? This is what companies like Celyad, Kyte Pharma and Novartis are doing: they extract living cells from patients or donors, modify them so they can detect and attack cancers and then re-inject them.
The figure below shows a simplified version of the process to change the cells. Obviously, step 3 groups several actions to change the cell characteristics.
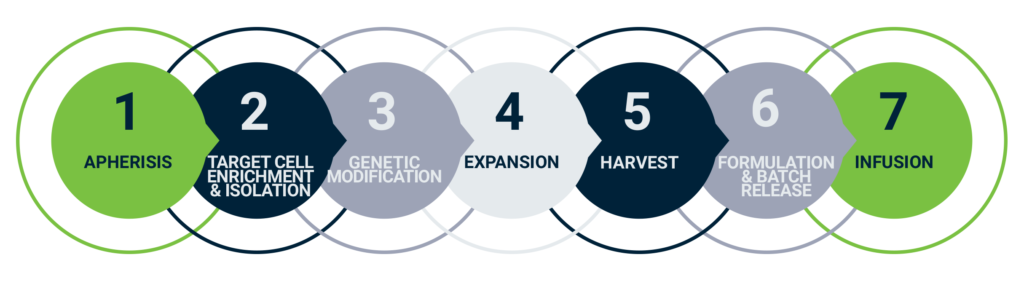 Autologous Car-T cell therapy workflow, adapted from evolution-bio.com
Autologous Car-T cell therapy workflow, adapted from evolution-bio.com
Now, if I tell you that 40% of us will get cancer in our lives and that Celyad has cured and “vaccinated” ovarian-tumor bearing mice using their NKR-2 T cells, you will probably understand where all this optimism is coming from.
State of things
So where are we today?
There are 2 types of cell therapy that have been around for years: blood and stem cell transfusion. Besides that, only a handful of therapies have been approved today. The first 3, and most famous ones, are Kymriah®, Yescata® and Provenge. Another famous treatment is Zolgesma. All of which will pop up again later in this article.
Still, it is safe to say that cell based therapy remains an emerging field of science with a lot of iterative learning. Currently, over 500 treatments are in phase 2 clinical trials, which has led to more attention from the market on 2 fronts. Firstly, several major investments (+1B€) by blue chip pharma have made the news in the last few years. Secondly, the regulatory requirements have grown and rightfully so:
- Changing cell characteristics often requires the use of viral vectors, which brings with it a very severe biohazard risk.
- Cell therapy products are more complex when compared to traditional therapies, with risks that may differ according to the type of product. The variability of the product, caused by starting material variation, complex manufacturing steps and challenges related to testing, leads to the need for a tailored regulatory approach.
As a result, the need for specific regulations is clear, but there are aspects that make it tricky.
As we’re talking about cells, the final product will never be exactly the same, which makes it difficult to describe and characterize the product. The most commonly adopted solution to tackle this, is to certify the process to create the product, rather than the final product itself. You will hear ‘the process is the product’ being dropped in lots of conversations and articles.
Now this adds a lot of complexity to an already challenging story. For example: what if you need to change your process along the way in order to avoid a price of 2 million euro per treatment?
Challenges
While the title clearly says challengeS, it all boils down to one major challenge: lowering COGs (cost of goods). The goal is not even to make the treatment cheap, just cheap enough to reach the reimbursement point. And that reimbursement point is very indication specific.
One of the first approved therapies, Kymriah, has a price point near 400K€ per treatment. At first sight, you would not expect it to be reimbursed, and yet it is and with good reason. Kymriah treats childhood ALL, a rare type of blood cancer, where the existing treatment has a 90% success rate. The remaining 10% are about 500 very sick children per year (in the US) and 70 Belgium (where I am from). Kymriah can help these patients and has a success rate of 83%. So for an amount that is manageable for the healthcare system, you can cure a relatively large number of children and young adults that would have definitely died otherwise. Still, 400K€ per treatment is high and any reduction would be welcome.
A counterexample is Provenge, the first approved immunotherapy that treats prostate cancer. Obviously, the target group for prostate cancer is much higher than ALL. On top of that, the treatment costs 93K€, and only prolongs life expectancy with about 4 months. This combination has led to Provenge not being reimbursed.
Another example is Zolgesma. With a list price of 2.125M€ per dose it is the most expensive drug in the world. Zolgesma is not yet being reimbursed in Belgium and hardly any people can free up that amount of money. Recently, Zolgesma got a lot of media attention when the parents of baby Pia started a crowdfunding to pay for their daughter’s treatment. While Belgians managed to gather the full amount in 2 days, there was a lot of discussion on the price of the drug and the fact that it is not being reimbursed.
One of the first questions I am sure everybody is asking: How do these products end up being that expensive? Is it really some big pharma exec saving up for a third yacht? To be sure, we dove in the main cost drivers (shown in the pie charts below).
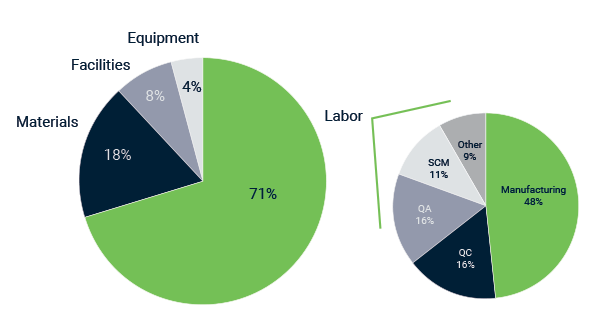 Main cost drivers in cell-based therapies, adapted from insights.bio
Main cost drivers in cell-based therapies, adapted from insights.bio
1. Labor cost
By far the largest contributor to the COGs is the cost of labor. Accounting for over two thirds of the total price tag. Of this portion, approximately half is manufacturing related, and the other half to various aspects such as quality control, quality assurance and supply chain management.
For now, we’re zooming in on: “How can one single treatment take this much manufacturing time?”.
Cell therapies are still an emerging field and the learning process tends to be iterative with lots of tweaking and testing. As a result, researchers end up with a complex and lengthy production process that is very treatment specific.
It is not surprising that such processes are failure prone. As a supplier, you would need to absorb these failures. “Listen patient X, you’ve paid us 250K for this treatment, but something went wrong so pay us another 250K”, is not an option. So suppliers calculate these failures in the final price of the treatment.
Why don’t they just come up with a better process then?
I told you earlier that, from a regulatory viewpoint, the process is the product. This means that the way to create the treatment is validated, not the treatment itself. On top of that, the process differs greatly from treatment to treatment. As a result, most steps towards automation are customized to one specific treatment and therefore expensive.
Now I am sure you will understand that investors are reluctant to invest large amounts in automation before a treatment is clinically validated. And by the time it is validated, the automate that you then want to build will have to do an exact repetition of the complex, manual process. Otherwise you have to re-do the clinical validation, which will lead to a later market entry.
Also not helping: Start-up exit strategies are often focused on getting acquired by big pharma companies (ideally for billions). Creating a treatment that works is more important than how that treatment will be produced.
Before we dive in automation as a solution, I will list the other major cost drivers.
2. Quality Assurance (QA) and facilities
You want a process that reduces risks and the main risk is contamination.
As a general rule: if you are worried about contamination, sterilization is the answer. Obviously, this is not possible if your product consists of live cells that would die as well. So for cell therapies there is a lot of focus on making sure contamination cannot occur.
To reduce the risk of contamination (of the sample as well as the outside world), the production of cell based therapies is done in a strictly controlled environment. These GMP compliant labs have very high demands on premises, equipment, training, personal hygiene of the staff and detailed documentation procedures for each step. The cost to build and maintain such a lab makes up about 8% (and up to 50%) of the total COGS of the cell based treatment. Steps to get you out of such a lab, or at least in a lower grade lab, would greatly help to reduce costs. A fully closed system that requires no direct interaction of an operator with cells and that controls temperature, CO2, humidity and much more, would be perfect of course.
While the cells themselves cannot be sterilized, you will need to sterilize or replace each part of the system that was in direct contact with cells during the manufacturing process. Otherwise you risk contaminating the batch of cells for the next patient. In this day of climate change awareness, sterilization seems preferable but is rarely the most reliable option and often not an option at all. It is not just about taking the steps to clean and sterilize parts, you will also need to validate that the sterilization was successful. For parts like filters, this is next to impossible.
This causes you to end up with thousands of euros in single use parts. Hence the 18% contribution of materials in the total cost of a treatment.
3. Quality Control (QC)
Once you have established a process that reduces risks, you will still want controlling mechanisms to see when anything goes wrong. And again, cells being cells, it is extra complex.
First of all because of the cost to make them. Simply taking a sample for QC and throwing it away is not preferable. Especially because you will need to repeat this QC step a bunch of times during an often lengthy process. You will end up throwing a large part of your precious cells away. So ideally, you move towards a nondestructive control of the cells.
With a production process this expensive, you also want to avoid continuing work when the cells are already lost. You would want a continuous view on cell growth throughout the production process so you can take immediate action. When your goal is to evolve towards a closed system, integrating an off-the-shelve microscope or cell imager is difficult.
A COTS (commercial off the shelf) microscope that meets your requirements may be available, but will be expensive and often overspecced for your intended use. On top of that, modifications will be required to get the right optical alignment and the necessary imaging quality. A custom microscope is worth exploring when the COTS microscope you can use, is very expensive or when you plan to make lots of them. You will be confronted with non-recurring engineering cost to design the new microscope, but will get important advantages: it is specced and designed for your specific application and the cost per unit is lower than a COTS solution.
4. Supply chain management
This should be easy! You just solved all the previous issues and complexities so what challenge can logistics offer you?
Quite a lot apparently:
Current therapies use cells from an individual patient to create a treatment for that same patient. As a result, scaling production offers no real value. The personalized nature of the treatment requires a scale-out of the production facilities, rather than production itself. This comes at a higher logistics cost and complexity as there is no economy of scale.
Traditionally, pharma uses a make-to-stock supply chain model. This is not applicable in the current cell therapies that are (currently) made-to-order. This pushes smart stock management off the table and leaves little to no margin for error.
So you end up with 3 choices, all of which with their own challenges:
- Centralized production in combination with transporting the cells from and to the patient is difficult because of the cell shelf life.
- Centralized production in combination with transporting the patient near production can be difficult because of the state of the patient.
- Producing the treatment near the patient is difficult because each manufacturing site needs to uphold the same high standards.
We have been talking about cell logistics, but it not easier for the raw material needed during the manufacturing process. Using a viral vector for transduction will not only have an impact on the production process, but also add logistical challenges as the viral vector supply has proven to be a capacity bottleneck in the past.
Opportunities for improvement exist at every stage of supply chain management, from integrated planning tools to distributed manufacturing models. The needs will be highly dependent on the process, but also on the treatment itself (maybe your patients are still able to travel, for example).
Solution to reduce the COGs: automation
I’ve mentioned why investors are reluctant to invest in automation before clinical validation of a treatment. But let’s say that has been tackled and you have found a lot of money: automation clearly is the way the go to:
- Decrease operator variability
- Increase operational efficiency
- Improve product consistency
- Reduce loss
- Reduce cost
How will you tackle this automation?
1. COTS automates
What? There are COTS automates?? Then why did I make you go through all this text? Because they are not perfect for each situation.
Two possible integrated automates are Miltenyi’s prodigy or Lonza’s cocoon. Both aim to reduce labor cost in manufacturing while minimizing the risk of contamination by developing a fully integrated ‘GMP-in-a-box’ automates.
Without the need for sterile connections in between process steps, the process can remain closed from start to finish without any contamination risk.
Choose these automates when:
- They can handle your process,
- You do not expect changes in your process,
- Your business model allows it,
- You do not mind depending on one single third party for your complete production.
2. Custom automates
One tip: never start designing the full system before you know all the building blocks will work.
Automating process steps separately, known as the ‘build a bear approach’, will give the manufacturer more independence from a single technology and allow them to focus on optimizing one step at a time, while the process continues to be optimized throughout development. If one piece of equipment fails, a back-up could be used without impacting the following process steps.
Sounds good. But what about the increased risk of contamination? Since scaling up is not an option in an autologous setting, the scale out approach requires the same amount of sterile connections regardless of the total volume to be manufactured on a yearly basis. What if you want to work in a decentralized, or even point-of-care setting, ideally in controlled but non-classified rooms?
The added complexity of GMP-in-a-box type equipment will require robust and reliable processing but enable lower-risk scale out. This trade-off depends on the maturity of the product, which is why the build-a-bear approach is having most traction in the, currently still relatively young, cell therapy industry.
Recently, an estimation was made for the ‘best case’ COGs with an optimized and automated process.
It took into account lean manufacturing, efficiency gains through digitalization, a 50% drop in infrastructure cost and a 30% drop in material costs. All these efforts together got the COGs per dose down from an estimated 58.200$ to 21.400$. An impressive improvement that will make cell therapies more accessible.
Still, when you add typical pharma margins to this amount, we end up with a market price of +100K$. That’s still too high for a wide roll out of treatments (source).
So is it all for nothing?
No, the steps towards automation are necessary. They are just not sufficient to make cell therapies accessible for everybody. To achieve that, a totally different path needs more exploring. Most cell therapies today use cells from a patient. These cells are then modified and re-injected in the same patient. This is called an autologous approach. To really unlock the full potential of cell therapies we need to evolve towards an allogeneic approach where cells from one donor patient are modified and then injected in tens, hundreds or thousands of patients.
We’ll get there, just not right now.
Download the perspective
Looking for solutions to innovate?
Leave us your email and get in contact with Wouter Hendrickx to help you with your innovation process.




