The majority of all medical innovations fail to enter the market due to human rejection. Even though the innovative solution has proven to offer increased efficiency, effectiveness and even cost reduction, the decision maker or end-user is very often not convinced of using it. Even after multiple formative usability studies in Human Factors and eliminating use-related hazards and usability problems the adoptability isn’t there. What could have gone wrong?

Human Factors Engineering is a very pragmatic and holistic process that helps innovators in the validation of medical device concepts. Though we notice that this approach is a risk-focused quality process which is very much regulated and focuses mainly on elimination of (serious) harm. That’s great, yet not enough (anymore).
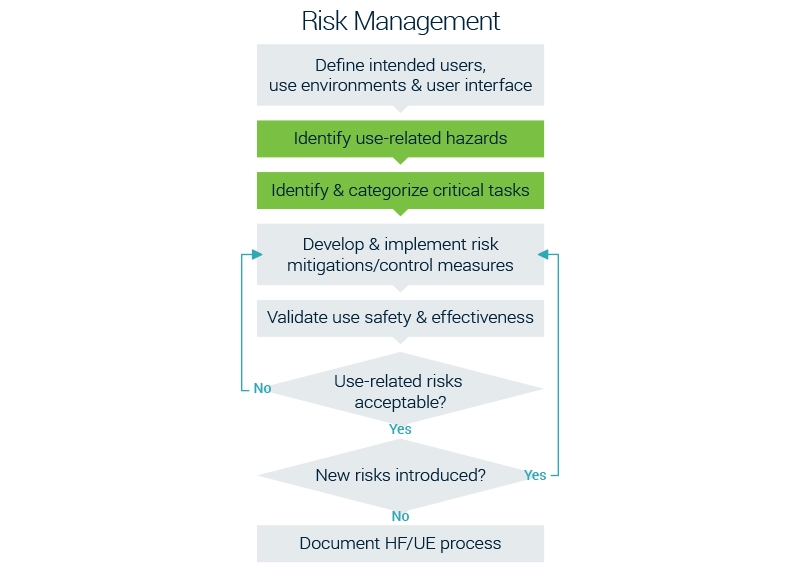
Root cause
We see that the root cause of many of these market failures is found in insufficient behavioral research. The analysis of how a user behaves towards a product is essential. It allows designers to create a product that fits in or positively transforms the current usage flow. Only by identifying how your product will influence a certain behavior you’re able to build meaningful interactions.
Therefore we believe that there’s a crucial risk-factor missing in the current HFE approach: de-risking resistance and rejection. Let’s take a look at this visual covering – what we believe – the 3 essential user-related high-level requirements. We like to rename it: the Human Behavior Engineering framework.
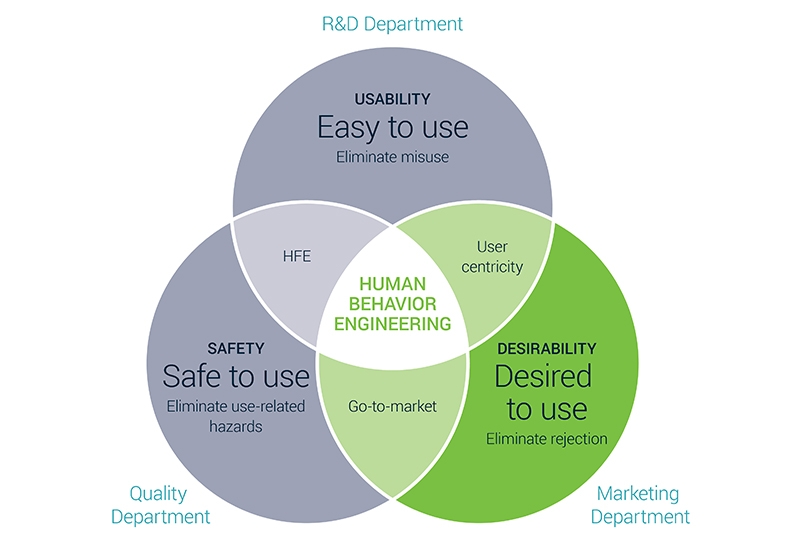
HFE typically lies in the overlap of safety and usability, where the FDA puts ‘safety’ conceivably higher than ‘usability’. Their guidelines tell designers to make sure that the design process validates features that eliminate use-related hazards and types of misuse (because of the improved usability leading to less hazards). We believe this is not enough to have a successful market entry.
Therefore we add a third component to the framework making sure to also validate market success. You need to search for desirability differentiators in your medical market by looking at the use environment, user profiles, interfaces and behavior.
A true fit-to-market medical device eliminates unintended use, use-related hazards and rejection.
By doing contextual inquiries and observations you’ll understand all external factors that might influence human machine interfaces. During these insightful activities, you have to search for hidden needs. Needs of which the users themselves didn’t know they had them. Features that the users don’t miss and really appreciate that they’re there. This is how we believe a product becomes meaningful.
Ignoring one of the 3 components can lead to an incomplete validation process. A true fit-to-market medical device lies in the sweet spot combining the 3 design objectives: eliminating unintended use (misuse), use-related hazards and rejection.
What happens when AI comes in?
Besides understanding the current behavior and searching for hidden needs, emerging technologies are used to change behavior. Digital health and Artificial Intelligence are changing our way of working which requires behavioral changes from multiple stakeholders. It’s risky because of the duality with their habits and familiarities. Especially in the medical industry, where many stakeholders are still very conservative.
Let’s give an example that everyone can relate to: self-monitoring your health through wearables.
Healthcare is changing from curative to preventive. This is an evolutionary shift which is made possible by for example the emerging technology of the estimation of blood pressure in smart watches combined with AI algorithms searching for patterns and predictions. Having all this data about your patient is great, but it also asks for a behavioral change from the patient’s and physician’s side. Are they ready for this? Who will become responsible: patient, physician or the device manufacturer?
By understanding the effect of design changes you’re able to create meaningful products that fit the current behavior (incremental change) or even develop a totally new behavior (disruptive change). Artificial Intelligence is a good example of completely disrupting the current behavior in the medical industry.
From risk management to risk & opportunity management
From the recommended HFE Risk Management approach showed at the beginning of this article we’ve reformed it so that it covers all important aspects including business success.
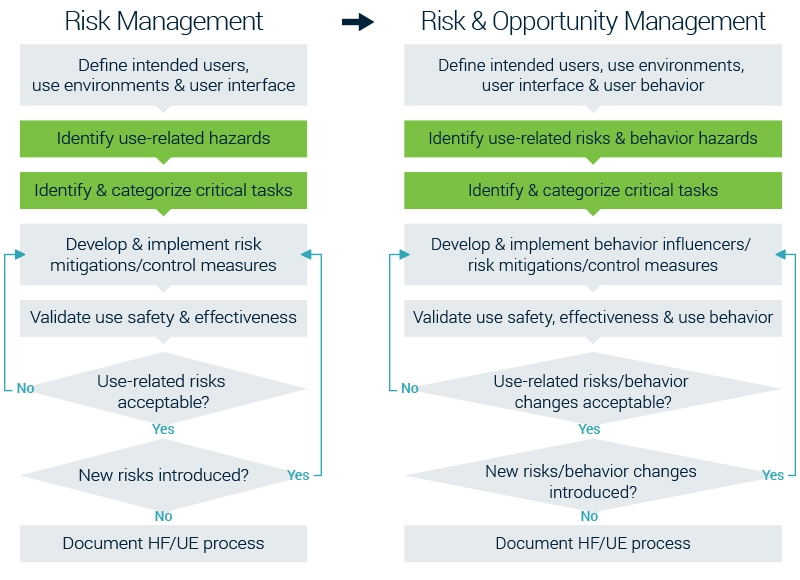
Risk management stays extremely important in the development of medical devices. Yet to achieve business success it’s key to create valuable products which will be adopted by your target audience. Therefore we see Opportunity Management as equally important as Risk Management. The combination is easily made and you’re not only validating your design concepts on risks but also on opportunities.
The reviewed Human Behavior Engineering approach will guide you in the development of new meaningful products. Bringing Human Factor Engineering to attention in your company benefits more departments than only R&D. You avoid complaints (customer support), eliminate post-market recalls (business), and you don’t get sued (legal). It’s a win-win for your business in general. Let’s start implementing!
This perspective is about being ready for future challenges in the adoptability of your next innovations and making sure to maintain business success in the medical industry. Make sure to fail fast and validate more for better. And remember to always search for the reasons for adoption as well as the reasons for rejection. Happy to discuss more on this topic!
Download the perspective
Download the study logic on behavioral design beyond human factor engineering




